
PharmaWatch™ Continuous Improvement: Alert Management
PharmaWatch™ Continuous Improvement: Alert Management By Michael RusnackVice President of Science and Engineering A large percentage of the work accomplished by the PharmaWatch™ development team
PharmaWatch™ is a software solution designed to deliver the best environmental monitoring and compliance reporting for the healthcare industry, and it can be used just as effectively whether working at home or in the office.
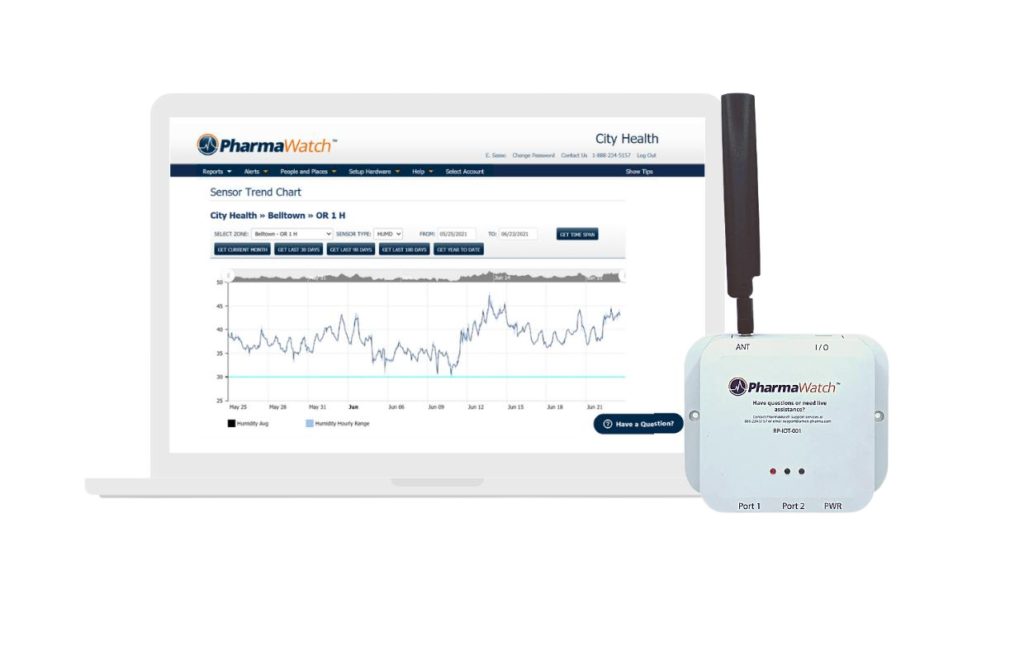










There are 5 major reasons that we are different than our hardware-sale motivated competitors.
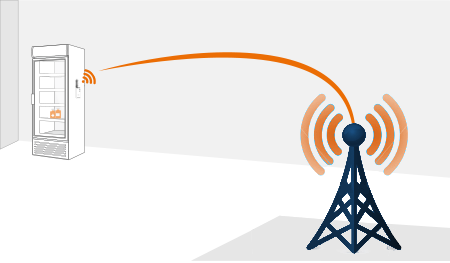
By utilizing multi-carrier/multi-bandwidth cellular communication, PharmaWatch™ Direct bypasses any connection to your private network. As a result, we are not a security risk at all. There is absolutely no chance that PharmaWatch™ can be used as an attack vector on your network. Furthermore, we will continue to report data when other solutions will not. Loss of AC power, WiFi connectivity, or Internet access at the monitored facility will not affect the sensor radio’s operation. We will continue to collect and report data for up to 5 days on battery alone. With regard to data security, PharmaWatch™ stores your critical data redundantly in the cloud across multiple data centers and geographic locations virtually eliminating risk of loss

PharmaWatch communicates via the latest IoT cellular technology. This means no extra hardware and virtually no setup required. The sensor radio will be delivered pre-configured to monitor the storage and handling of your COVID-19 Vaccines according to manufacturer specifications. Best of all, they are plug-n-play!

Our devices can monitor a wide range of parameters include differential pressure, CO2, humidity, and temperatures from -200°C (-328°F) to 50°C (122°F). Applications include:
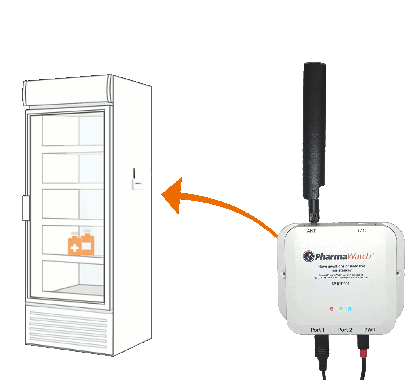
Once the probe is installed into the monitored environment and connected to the PharmaWatch™ Direct sensor radio, you are ready to go. Nothing more is required. The sensor radio is pre-configured to communicate with the PharmaWatch™ Portal via the Cell Network using multi-carrier/multi-bandwidth communication, PharmaWatch™ Direct bypasses any connection to your private network, without any further configuration or changes. It’s that simple.
Don’t just take our word for it. See what our customers have to say.

PharmaWatch™ Continuous Improvement: Alert Management By Michael RusnackVice President of Science and Engineering A large percentage of the work accomplished by the PharmaWatch™ development team
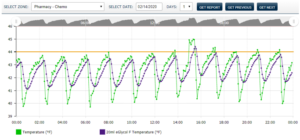
Virtual Temperature Buffering By Michael Rusnack In the Spring of 2012, the PharmaWatch development was made aware of the Center for Disease Control and Prevention
This post has Moved! Follow the link to view: https://www.pharmawatch.com/covid-19-vaccine/your-current-monitoring-may-not-be-good-enough-for-covid-19-vaccines/
Fill out the information below to start your FREE demo.
