- NIST Certificate included at no additional cost and valid for two years.
- We have a variety of probe types to accommodate every need
- Temperature
- Humidity
- Differential Pressure
- And More…
Not sure which probe will work for you? No problem, we help select the required probes based on your unique needs.
At the click of a mouse, PharmaWatch™ provides a streamlined interface with State Agencies as well as full documentation. It is also automatically updated whenever a new Federal or State compliance regulation is added or amended.
Planned or not, audits consume time, disrupt work routines and drain valuable resources. The advanced reporting and analytic capabilities of PharmaWatch automate the process. This insures a painless, best-practices response to the most rigorous audits.
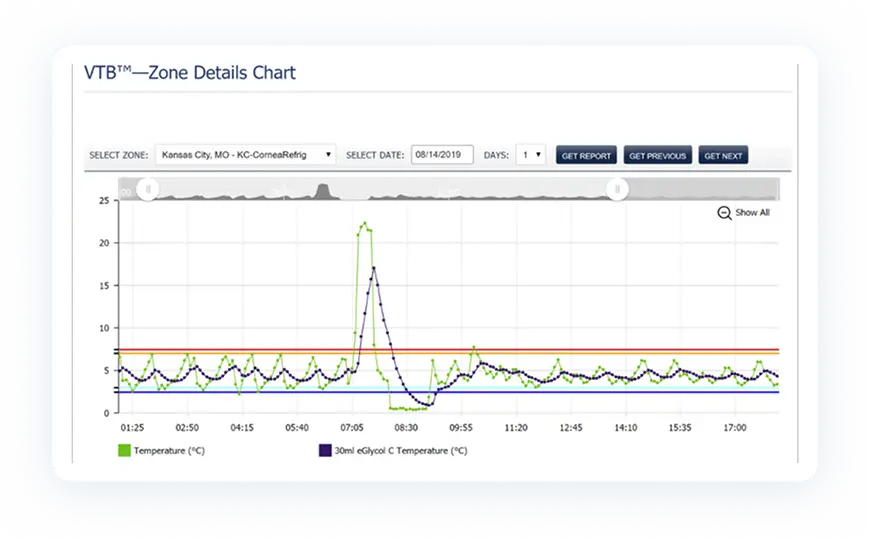
We not only monitor your critical environments, we simplify and automate the auditing process. Our solution frees you and your staff from the time-consuming and error-prone task of manually recording temperature, humidity and other readings and keeping track of all that historical data.
All PharmaWatch™ reports and records are fully FDA 21 CFR part 11 compliant. They are securely stored on virtualized fault-tolerant systems and instantly accessible whether you need the report from yesterday or one from years ago. (FDA CFR part 11 specifically regulates the secure creation, storage and management of electronic records under an electronic signature policy for data compliance.)