The Requirements
COVID-19 Vaccine Storage and Handling
Here you will learn more about the CDC’s requirements for proper storage, handling, and transport of your COVID-19 Vaccines.
Call 1 (888) 234-5157 now and speak with one of our monitoring experts
The Requirements
Here you will learn more about the CDC’s requirements for proper storage, handling, and transport of your COVID-19 Vaccines.
AmericanPharma's work with vaccine storage and handling is really innovative.
Health Advisor - CDC Senior Public Health
While storage and handling Guidances for COVID-19 Vaccines have not yet been published by the CDC, current guidance on “Vaccine Storage and Handling” continues to support “if the cold chain is not properly maintained, vaccine potency may be lost, resulting in a useless vaccine supply.”
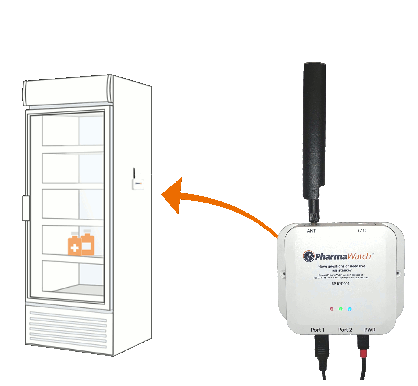
To avoid this, those overseeing distributions must know, without a doubt, which vaccines are viable, and which are not. PharmaWatch COVID™ was purposely built as a solution to this problem for COVID-19 vaccines. PharmaWatch COVID employs a real-time, continuous monitoring IoT sensor that gathers and reports data at every step in the supply chain. As data is received, it is analyzed in real-time via proprietary algorithms which help detect and alert on any conditions which could compromise the safety and efficacy of COVID-19 vaccines.
mRNA COVID Vaccines requiring Ultra Cold (<-70C) storage conditions pose a unique challenge to traditional vaccine cold storage and cold-chains. PharmaWatch COVID was designed specifically for this challenge and is the most scalable and cost-effective Ultra-Low Vaccine temperature monitoring system available.
Requiring no data backhaul infrastructure (e.g. no routers, range extenders, or server infrastructure), PharmaWatch COVID can be deployed more rapidly and effectively into COVID-19 Vaccine Ultra-Low Temperature Storage environments than any other solution on the globe.
The only cold storage vaccine monitoring system that exceeds the recommended compliance and safety requirements of your COVID-19 vaccines.
PharmaWatch COVIDTM communicates via the latest IoT cellular technology. This means no IT services to consult, no infrastructure to install, and a solution running on the largest and most secure IoT network in the nation.
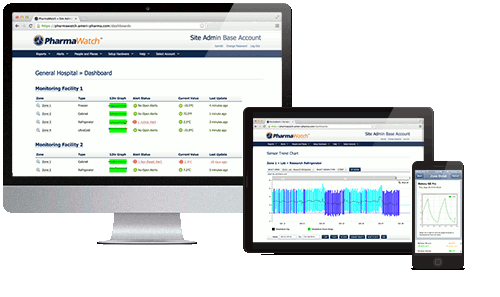
Monitor your vaccine storage and transport conditions, anywhere, at any time, on any device.