
Celebrating Over 15+ Years In Business
15 Years of Protecting What Can’t be Replaced This year marks an important milestone for PharmaWatch® — 15 years in business. While our technology has
Call 1 (888) 234-5157 today and speak with one of our monitoring specialists
Trusted Globally 15+ Years
PharmaWatch™ delivers FDA CFR Part 11 and GxP compliant, real-time monitoring for temperature, humidity, pressure, and liquid nitrogen storage, with audit-ready reporting and automated compliance in one secure platform.
Ready to eliminate monitoring headaches and pass every inspection?





















There are 4 major reasons that we are different than our hardware-sale motivated competitors.
PharmaWatch™ uses secure, multi-carrier cellular communication—completely bypassing your internal network. That means no exposure to your firewalls, no risk to your IT infrastructure, and no attack surface for bad actors.
Even if power, Wi-Fi, or internet access fails, your monitoring doesn’t. PharmaWatch™ continues collecting and transmitting data for up to 5 days using built-in battery backup.
To ensure data integrity, all information is stored redundantly across multiple secure cloud locations—virtually eliminating risk of data loss.


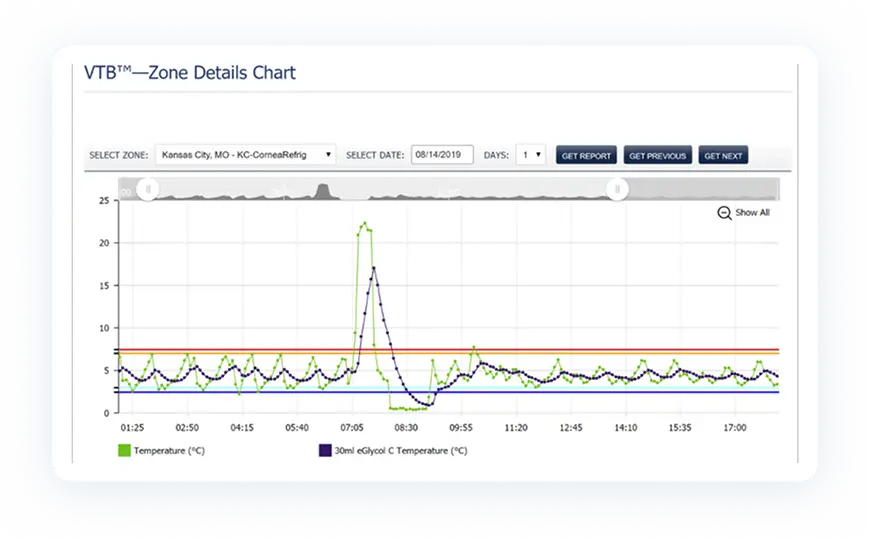
PharmaWatch™ automatically captures all required audit documentation, including FDA CFR 21 Part 11 and GxP compliance, as well as CDC/NIST-aligned monitoring records. With our OneClick™ audit support tool, generating inspection-ready reports takes seconds—not hours—keeping you confident and compliant at all times.
PharmaWatch™ goes beyond traditional data loggers with continuous temperature and humidity monitoring, intelligent alerting, and automated compliance reporting—all accessible from one centralized, cloud-based dashboard.
No manual downloads. No IT setup. Just always-on monitoring that keeps you audit-ready and in control.
Best of all, it’s truly plug-and-play!

We offer a flexible subscription model designed to fit operational and financial goals without requiring upfront capital investment. With no hardware to purchase and no IT burden, our enterprise-grade monitoring system helps you stay compliant while maintaining predictable costs.
Try it, scale it, or deploy it long-term—on your terms.
Fast setup. Zero IT. Instant peace of mind.
PharmaWatch™ arrives pre-configured and ready to go—no complex setup or IT support required. Simply plug it in and start monitoring temperature, humidity, or liquid nitrogen levels in minutes.
Your inventory stays protected from day one, with real-time alerts and audit-ready compliance built in.
Our devices can monitor a wide range of parameters include differential pressure, CO2, humidity, and temperatures from -200°C (-328°F) to 50°C (122°F). Applications include:

Once the probe is installed into the monitored environment and connected to the PharmaWatch™ Direct sensor radio, you are ready to go. Nothing more is required. The sensor radio is pre-configured to communicate with the PharmaWatch™ Portal via the Cell Network using multi-carrier/multi-bandwidth communication, PharmaWatch™ Direct bypasses any connection to your private network, without any further configuration or changes. It’s that simple.


Industry-first liquid nitrogen depth monitoring with real-time alerts.
Don’t just take our word for it. See what our customers have to say.

15 Years of Protecting What Can’t be Replaced This year marks an important milestone for PharmaWatch® — 15 years in business. While our technology has

Protecting What Matters: Introducing Wetness Detection by PharmaWatch® As the winter season settles across much of the Northern Hemisphere, moisture is never far behind. Rain,

Introducing Our New PharmaWatch® Refrigerator Sensor Installation Video We’re always working to make your monitoring experience clearer, faster, and more efficient. To support that mission,
Ready to eliminate monitoring headaches and pass every inspection?

Have more questions or need immediate assistance? Call 1 (888) 234-5157


To continue, please fill out the below information.


Thanks for reaching out — one of our experts will be in touch shortly to schedule your demo.