Unleashing the Potential of Virtual Temperature Buffering
Episode 4: Accepted By All Regulatory Bodies
The previous three episodes established that the need and value for temperature buffering are not just important but crucial. The primary objective is to accurately represent the temperature experienced by the storage unit’s contents. PharmaWatch™ Virtual Temperature Buffering is the only solution that effectively simulates the thermal properties of the items stored in the refrigerator, providing a more realistic measurement of the temperature conditions experienced by the stored products.
A common practice in monitoring cold chain conditions for temperature-sensitive products is to employ a physical thermal buffer into which the temperature probe is inserted. This buffer may be a bottle of glycol or other liquid, a container of glass beads, an aluminum block, or nearly any other media the user feels appropriate. The purpose of the buffer is to simulate the experience of the stored product rather than the air temperature. Simulated stored product will not be accomplished because the physical buffer does not match the stored product’s thermal properties and container.
Since 2012, PharmaWatch™ has committed to improving the quality of monitored materials and addressing the gap in temperature buffering. The first question raised was – does the size of the buffer matter? The standards and solutions available failed to address the question; thus, we committed to delivering a solution. Our first step was to assess precisely what was being monitored. It was noted that in the same storage unit contained volumes ranging from 5ml to 300ml. It became evident that a single-volume buffer would not work. The ideal tool would be a universally applicable buffer.
PharmaWatch™ developed a mathematical model that could simulate the temperature of any stored good—any volume, geometry, or material. The first technical paper was published in 2012. The effort resulted in two United States Patents being issued, which clearly recognized the novelty of the work.
The first implementation of the PharmaWatch™ glycol substitute was the simulation of a cornea chamber. This complex shape was simulated with Virtual Temperature Buffering, which was determined to represent the physical container with an accuracy of .999. During the patent development, filings, and prosecution period, extensive testing was performed comparing the model to its physical counterparts. This work developed an extensive library of constants that represent common shapes, such as the Boston Bottle, with multiple volumes of blood bags and tissue storage packages. The application of the technology has grown to simulate multiple volumes, from simple to complex shapes.
Clients and regulatory bodies have universally adopted and accepted this improvement to cold storage monitoring. In addition to demonstrating the solution in customer applications, the technology continues to be presented in peer-reviewed journals via manuscripts and presentations. The use of Virtual Temperature Buffering has become widely accepted in the pharmaceutical industry. States use this technology to monitor vaccines supplied by the Federal Vaccine for Children program. Hospitals, clinics, and other facilities have replaced physical buffering with the virtual solution provided by PharmaWatch™, improving the quality of care while reducing overall storage costs.
The same regulators that mandate temperature buffering suggested alternate materials; however, buffer methodologies, such as volume, were unclear. Virtual temperature buffering operates on the measured air temperature, leaving the air temperature unchanged. The storage unit temperature is monitored using two methods, air temperature and the simulated glycol temperature. This is shown in Figure 1 below.
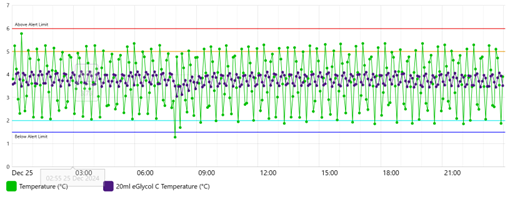
Figure 1 – Air Temperature and Virtual Glycol
PharmaWatch™ solutions exceed the regulatory requirements of agencies that oversee product storage. Virtual Temperature buffering has been adopted across the cold storage industries, maintaining many advantages, improved quality of material storage, reduced loss of expensive lifesaving materials, improved care, while reducing the overall costs to healthcare services. The next episode of this series will cover many of those advantages.
Written by Michael Rusnack, VP of Science at Engineering


