Arkansas Vaccines for Children Program
PharmaWatch™ helped administrators become aware and compliant in over 100 clinics with more than 400 separate storages scattered across the State of Arkansas. Patient safety was improved, vaccines were significantly less likely to be thrown away, and overall efficiency was increased for the state program.
ENSURING DELIVERY OF SAFE AND EFFECTIVE VACCINES BY HEALTHCARE PROVIDERS IS CRITICAL TO CONTROLLING PREVENTABLE DISEASES.
In 2012, the Office of Inspector General published a report that brought visibility into serious vulnerabilities in the management of vaccines. The Centers for Disease Control and Prevention (CDC) estimate that among children born in the last 20 years vaccinations will prevent more than 21 million hospitalizations and 732,000 deaths (CDC, 2014b). However, those impressive numbers would be even higher, says the CDC, if vaccines were better monitored during storage (CDC, 2014a). In a major report of a study involving 45 providers in five states who offered free immunizations as part of the Vaccines for Children (VFC) Program, the researchers found that 76 percent of providers stored their vaccines at temperatures that were either too hot or too cold (Department of Health and Human Services [DHHS], 2012, p. 14). According to that report (pp. 14-15), a percentage of vaccine doses were at risk due to inappropriate storage temperature and consequently “may not provide children with maximum protection against preventable diseases.” These reports motivated the healthcare industry to take steps to properly manage vaccines important to controlling preventable diseases. In addition to the challenge above, consider having over 400 vaccine storage units across 100 locations in your state and it is your responsibility to ensure safe and effective delivery of vaccines to the citizens. This is the challenge the State of Arkansas addressed, and the result had benefits well beyond the goal of delivering safe and effective vaccines.
COMPLIANT, SCALABLE, AND SECURE STATEWIDE VACCINE MANAGEMENT SOLUTION
The objectives were clear. Implement a solution that would comply with changing agency regulations that would seamlessly scale across the State, is easy to use, affordable, and secure, with minimal support costs.
In a three-way collaboration between the State of Arkansas, Johnson Controls, and American Pharma Inc. a state-of-the-art, cloud-based solution called PharmaWatch™ was deployed.
RESULTS
With all the data in a single database, PharmaWatch™ provides advanced algorithms and an intuitive user interface that was easy to learn and manage. Manually generated reports required for audits were automated, and staff was trained with minimal effort. Additionally, daytime alerts were reduced, and nighttime alerts were caught before vaccines were lost.
More Effective Capital Spending – In the past, it was a challenge to understand what equipment needed to be serviced or replaced. Now, Johnson Controls is able to monitor equipment performance and advise where the State’s dollars can best be spent to address the most critical needs. Visibility into where capital expenditures are needed has helped Johnson Controls create value and reduce costs for their customers. In addition, it is now possible to assess the performance of various equipment manufacturers to avoid spending dollars on equipment that may not perform up to industry standards.
Reduced and More Effective Service Trips – A single portal provides visibility to detailed reports for Johnson Controls and has minimized the number of service visits required within the State of Arkansas. Service visits that are required are now provided with better information concerning the nature of the problem. Engineers and technicians are often able to diagnose a problem before heading to the field, making the trip more productive and reducing costs for Johnson Controls and their customers.
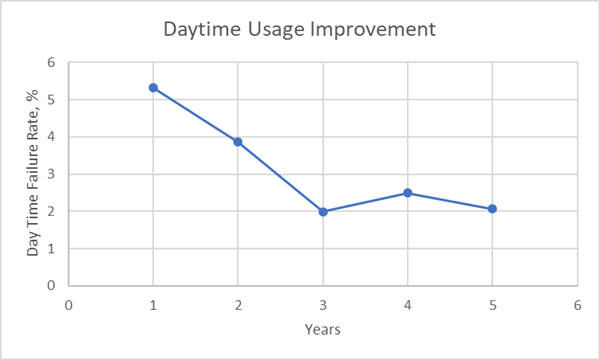
Reduction of Daytime Alerts – A service of the PharmaWatch™ solution is the training and awareness of preventable alert events. The typical “daytime” alert is a door open event. These events are associated with the storage unit door being left ajar or left open for a long stocking and inventory evaluation. Below you can see a significant reduction in daytime excursions over the course of five years, indicating that staff implemented better practices as a result of receiving PharmaWatch™ real-time alerts.
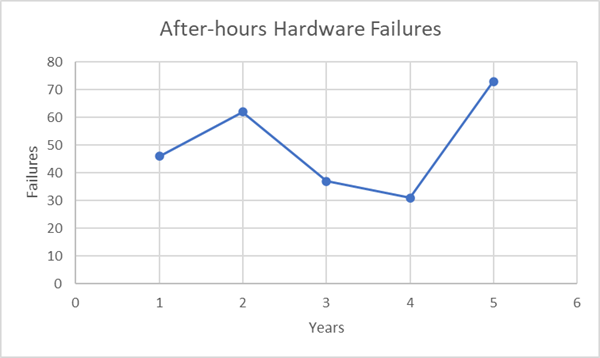
Awareness of After-Hour/Weekend Excursions – The occurrence of an after-hours/weekend alert is most likely due to hardware failure (no human intervention). An event at night would go unnoticed while monitoring with a data logger, which would result in a loss of inventory or decreased efficacy. PharmaWatch™ real-time monitoring provides alerts on time, allowing the client to address any conditions no matter the time of day or day of the week.
Over five years, more than 5,800 daytime- and nearly 275 after-hours alerts were recorded and addressed using the PharmaWatch™ solution. Without a monitoring solution, many of these failures would have resulted in a significant loss of inventory. With each unit storing an average of $50K of material, the PharmaWatch™ solution prevented the loss of over $13M in potential night-time or weekend failures. If employing dataloggers, staff would be unaware of the the night-time/weekend failures until the following day. At which point, most or all of the vaccines would have to be destroyed.
CONCLUSION
Cloud-based solutions, which were new a few years ago have proven their effectiveness and have enabled value creation for companies like Johnson Controls to provide to their customers. With all data in a single database, PharmaWatch™ is able to provide Johnson Controls and their customers proactive alerts to potential inventory loss, maintain a compliance solution across the State, and provide new features and reports, with significantly reduced support costs.
Instead of labor-intensive monitoring and documenting of vaccine storage temperatures, nurses can focus on the important client and patient needs and core operations. Today’s new technological innovations in continuous temperature and environmental monitoring such as with PharmaWatch™ will ensure the ability of nurses to provide patients with viable vaccines that have been stored and protected according to the highest safety standards. [RN], (2015)
