Just that easy
Effortless Installs, Zero IT Headaches
Our sensors arrive pre-configured, connect over cellular or wireless, and install in minutes—no Wi-Fi, no downtime, no stress.
Call 1 (888) 234-5157 today and speak with one of our monitoring specialists
Because PharmaWatch™ is delivered as a secure, cloud-based SaaS platform, there are no software licenses, no support fees, and no surprise upgrade costs—ever.
Once your system is installed, you’ll get access to easy-to-manage user logins, password controls, and electronic signature tools—all fully compliant with FDA 21 CFR Part 11.
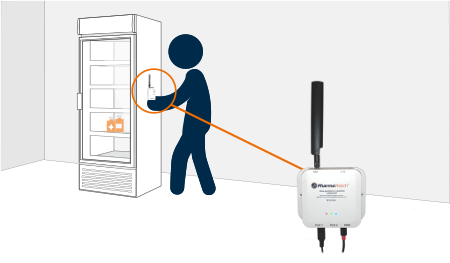


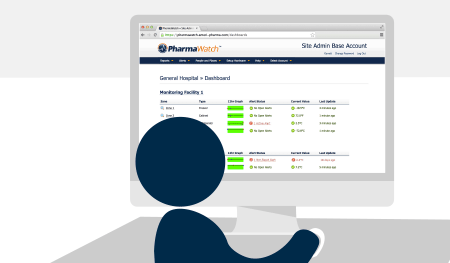
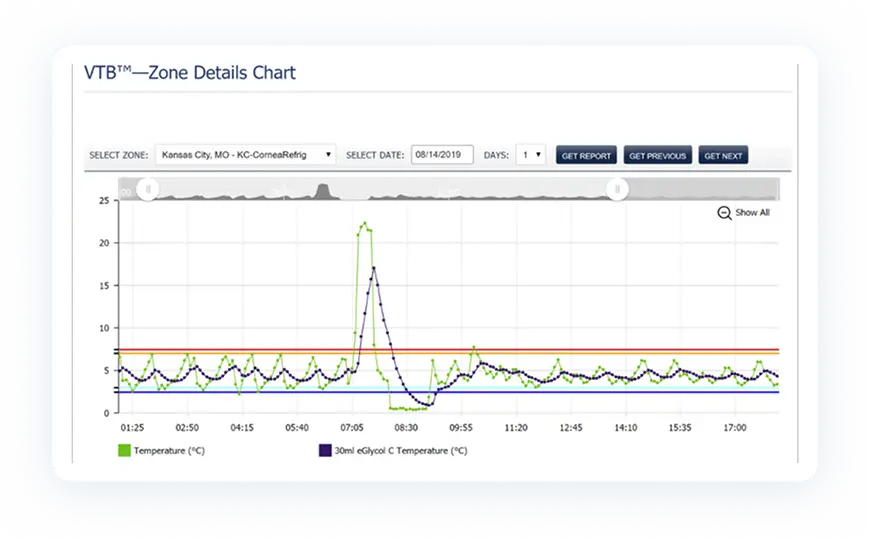
Once installed, PharmaWatch® begins transmitting secure, real-time data to the cloud via cellular connection. Sensor readings are validated using NIST/NPL-traceable reference thermometers, and all compliance documentation is auto-generated in electronic format.
Audit-ready. Secure. Simple.
Ready to eliminate monitoring headaches and pass every inspection?

Have more questions or need immediate assistance? Call 1 (888) 234-5157


To continue, please fill out the below information.


Thanks for reaching out — one of our experts will be in touch shortly to schedule your demo.