[fusion_builder_container hundred_percent=”no” equal_height_columns=”no” menu_anchor=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”center center” background_repeat=”no-repeat” fade=”no” background_parallax=”none” enable_mobile=”no” parallax_speed=”0.3″ video_mp4=”” video_webm=”” video_ogv=”” video_url=”” video_aspect_ratio=”16:9″ video_loop=”yes” video_mute=”yes” video_preview_image=”” border_size=”” border_color=”” border_style=”solid” margin_top=”” margin_bottom=”” padding_top=”” padding_right=”” padding_bottom=”” padding_left=””][fusion_builder_row][fusion_builder_column type=”1_1″ layout=”1_1″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”” dimension_margin=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no”][fusion_text]
PharmaWatch™ CFR 21 Part 11 Software Compliance
[/fusion_text][/fusion_builder_column][fusion_builder_column type=”1_2″ layout=”1_2″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”” dimension_margin=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no”][fusion_text]In March of 1997, FDA issued final part 11 regulations that provide criteria for acceptance by FDA, under certain circumstances, of electronic records, electronic signatures, and handwritten signatures executed to electronic records as equivalent to paper records and handwritten signatures executed on paper. The pharmaceutical & medical industry hugely relies on electronic systems and records. In order to comply with FDA regulatory requirements, they have to set up various processes and systems. This document describes how the PharmaWatch™ solution helps them comply with CFR 21 Part 11.[/fusion_text][fusion_text]
FDA ruling 21 CFR Part 11 specifies how electronic records and electronic signatures can be used as a substitute for paper records and handwritten signatures. It is broadly applicable to electronic records, including temperature measurement. The goal of this paper is to educate readers so they can understand the impact of this ruling and learn how to achieve compliance through the intelligent use of tools and process.
[/fusion_text][fusion_text]
What are electronic records?
[/fusion_text][fusion_text]
According to the FDA, an “electronic record means any combination of text, graphics, data, audio, pictorial, or other information representation in digital form that is created, modified, maintained, archived, retrieved, or distributed by a computer system.” Not all electronic records are subject to 21 CFR Part 11, only those that are maintained in accordance with FDA published predicate rules. These rulings, such as the Good Laboratory Practice (GLP) and Current Good Manufacturing Practice (CGMP), mandate what records must be maintained, what needs to be contained in the record, whether signatures are required and how long records must be maintained.
[/fusion_text][fusion_text]
What is an eletronic signature?
[/fusion_text][fusion_text]
Electronic signatures are intended to be binding digital equivalents of handwritten signatures. The FDA states that an “electronic signature is a computer data compilation of any symbol or series of symbols executed, adopted, or authorized by an individual to be the legally binding equivalent of the individual’s handwritten signature.” It is important to note the FDA does not equate an electronic signature with a digital signature, such as those provided by commercial entities Verisign, Entrust, etc. FDA predicate rules specify which electronic records require signatures, electronic or otherwise. If signatures are necessary, and they are collected electronically, then compliance with 21 CFR Part 11 is mandatory.
[/fusion_text][/fusion_builder_column][fusion_builder_column type=”1_2″ layout=”1_2″ spacing=”” center_content=”no” hover_type=”none” link=”” min_height=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” background_color=”” background_image=”” background_position=”left top” background_repeat=”no-repeat” border_size=”0″ border_color=”” border_style=”solid” border_position=”all” padding=”” dimension_margin=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=”” last=”no”][fusion_imageframe image_id=”12383″ style_type=”none” stylecolor=”” hover_type=”none” bordersize=”” bordercolor=”” borderradius=”” align=”center” lightbox=”no” gallery_id=”” lightbox_image=”” alt=”” link=”” linktarget=”_self” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” animation_type=”” animation_direction=”left” animation_speed=”0.3″ animation_offset=””]https://community.ameri-pharma.com/wp-content/uploads/2017/11/test-tubes.jpg[/fusion_imageframe][fusion_separator style_type=”none” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” sep_color=”” top_margin=”5″ bottom_margin=”5″ border_size=”” icon=”” icon_circle=”” icon_circle_color=”” width=”” alignment=”center” /][fusion_text]
How does the ruling impact temperature measurement?
[/fusion_text][fusion_text]
To meet the requirements of 21 CFR Part 11 a software project must follow rigorous change tracking and signoff procedures. The intent of this ruling is to ensure there is a clear and irrefutable record of each and every change made during the project lifecycle. This otherwise cumbersome task can be made simpler using change management tools that are built with compliance in mind. When selecting these tools one should look for the following qualities: • Access is limited to authorized users. • Records can only be updated by users with security access. • Timestamps are recorded for each and every change. • Changes are authenticated using electronic signatures. • Accurate change histories are maintained for all files. • Meets audit trail requirements. • Traceability throughout the entire lifecycle.
[/fusion_text][fusion_text]
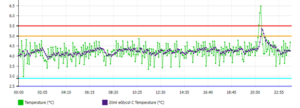
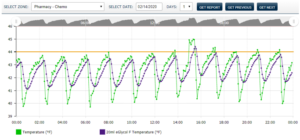
Fulfilling these requirements is straightforward with the PharmaWatch™ Web Portal which is built for the task and offer an array of features that ease regulatory compliance. PharmaWatch™ helps organizations meet FDA requirements with a capable temperature monitoring solution system that supports electronic signatures and audit trails (see Figures 1 and 2). The PharmaWatch™ Web Portal furthers regulatory compliance by accurately tracking changes to any digital — data, users, sensors.
[/fusion_text][fusion_fontawesome icon=”fa-file-o” size=”13px” flip=”” rotate=”” spin=”no” alignment=”” hide_on_mobile=”small-visibility,medium-visibility,large-visibility” class=”” id=”” circle=”yes” iconcolor=”” circlecolor=”” circlebordercolor=”” animation_type=”” animation_direction=”down” animation_speed=”0.1″ animation_offset=”” /][fusion_text]
Download the Whitepaper
[/fusion_text][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container]