ARe you Prepared?
Have questions? Call 1.888.234.5157 and we’ll be happy to help.
What We Know
At least one of the COVID-19 vaccines will require storage in ultra-low temperature conditions. Recent news has pointed to the fact that Pfizer’s COVID-19 vaccine will require storage at -94°F. These vaccines will be shipped in dry ice containers, likely with a data logger to monitor the temperature along the way. Upon arrival, monitoring will be the responsibility of the jurisdiction or vaccination site. According to the CDC COVID-19 VACCINATION PROGRAM INTERIM PLAYBOOK, “Every vaccine storage unit/container must have a temperature monitoring device.” This is true for ultra-low storage as well as refrigerators and freezers. The CDC’s current recommendations are for the use of a Digital Data Logger (DDL) for this purpose, but that is not enough to ensure viability of COVID-19 vaccines and the safety of those receiving it.


Why a DDL Is Not Enough
- DDL’s require periodic check-ins through visual inspection to check battery life, available storage, and any exceeded alert conditions.
- Needs physical removal from storage unit to recover data – this temporarily suspends monitoring in order to review and respond to any excursions
Because of the nature of this technology, the use of a DDL to monitor vaccines is purely reactive. For example, if a temperature excursion takes place during off-hours, you won’t be made aware until the next morning, when the efficacy of your COVID-19 vaccines could already be in jeopardy. With a real-time solution, you will be remotely notified of any temperature excursion so that you can tend to your vaccines BEFORE they become inviable.

The Best All-in-One Monitoring Solution Out There
PharmaWatch utilizes the most accurate technology available for refrigerated, frozen, and ultralow conditions. And we do it with the lowest total cost of ownership.
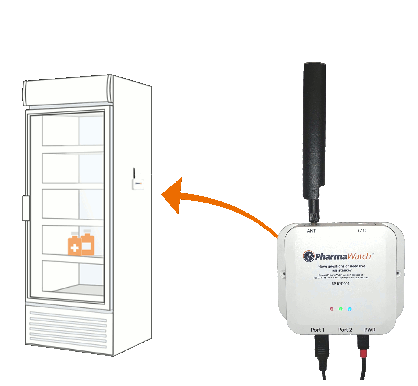
Monitors Continuously – PharmaWatch monitoris your vaccines around the clock, reporting data to the portal every 5 minutes. Due to the utilization of LTE Cat M1 cellular, our sensors will continue monitoring and reporting through a power outage, WiFi network failure, or during transportation.
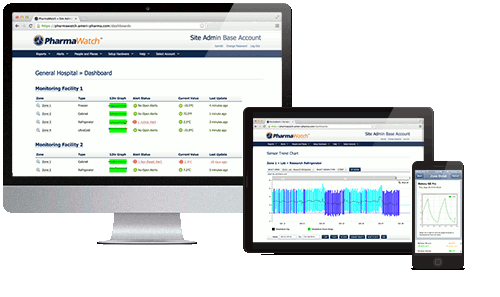
Automated reporting & check ins – Vaccine managers can view the status of each monitor, complete daily check-ins at the click of a button, and print fully compliant audit reports, all from the PharmaWatch portal.