In previous blogs, I have cited the Center for Disease Control and Prevention – Vaccine Storage and Handling Toolkit. Below is the first paragraph in the introduction of this document.
“Proper vaccine storage and handling are important factors in preventing and eradicating many common vaccine-preventable diseases. Yet, each year, storage and handling errors result in revaccination of many patients and significant financial loss due to wasted vaccines. Failure to store and handle vaccines properly can reduce vaccine potency, resulting in inadequate immune responses in patients and poor protection against disease. Patients can lose confidence in vaccines and providers if they require revaccination because the vaccines they received may have been compromised” (CDC, 2020).
The message outlined in the introduction lays the groundwork for all we work to accomplish here at PharmaWatch™. As stated previously, we utilize this guidance as to the means for the development of our solutions and product offering. It is our goal to provide an answer that not only meets, but exceeds the objective outlined above. Throughout this blog series, our intent is to bring greater clarification to the guidance provided by the CDC.
Digital Data Loggers
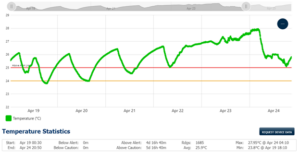
Key to the proper storage and handling of vaccines, blood, plasma, pharmaceuticals, tissue, and other temperature-sensitive materials is the monitoring equipment. The CDC guidance in this document recommends a specific temperature monitoring device called a digital data logger (DDL). The DDL has the capability to record the temperature at fixed intervals, sample every 30 minutes, for example, store thousands of readings, and indicate visually if the storage unit has exceeded the preset control limits.
The data from these devices can be downloaded to a computer using special software or retrieved from a website. The recorded data will allow the user to look back over a time period to determine the viability of the stored good as it may have been affected by a temperature excursion.
The use of the DDL requires a person to:
Periodically check the DDL state through visual inspection – AKA Twice daily check-In
- Battery low indication
- Memory full indication
- Alert condition exceeded
Physically remove the DDL from the storage unit to recover the stored data
- Suspend monitoring during the data recovery process
- Review the stored data and interpret the results
- Respond to any anomalies or excursions noted
This solution results in a purely reactive mode when monitoring stored goods, by which an excursion is only noted after the fact and only then is acted upon by the user. The time period can range from a few minutes to days depending on the staffing.
When considering a monitoring solution, the cost of the DDL is not the only factor to take into account. There is the human factor that is required to inspect the logger state daily, download, and interpret the data. Given the reactive nature of this type of solution, the possibility of the stored goods being spoiled due to a temperature excursion must be considered as well.
When the data logger was chosen as the solution, it was likely the best available technology at the time. Cloud storage, integrated WiFi, and now low-cost cellular solutions are enabling the real-time collection, storage, and management of the data. Furthermore, with Cloud Computing and Storage the data can be accessed from a web browser simplifying the process and providing real-time analysis to the user.
The PharmaWatch™ Solution
The PharmaWatch™ solution communicates directly from the device to the cloud. There is no need for additional software or servers. Users access their data via a web browser as simple as logging into a favorite website. After logging in to the PharmaWatch™ portal, the user can view the status of each monitor at a glance, all in one place.
When required, formatted data is available in the form of summary reports that are easily accessed, viewed, and printed. Also stored within the portal are the calibration data and NIST traceable certifications for each device. These documents are available in real-time and can be accessed in three clicks or less. Furthermore, all documentation is audit-ready and fully compliant. When requested for an audit, there is no fumbling or searching; each certificate is stored with all necessary information.

The PharmaWatch™ team has made access available to the same web-based information on a mobile app. The app is available for smartphones and tablets and can be accessed anywhere, anytime.
Where the data logger requires the user to access the logger to determine the state of the storage unit (i.e. any alert condition that may have occurred), the real-time solution can be configured to notify the user(s) in the event of a fault condition. These include, but are not limited to temperature excursion, communication loss, power loss, battery charge state, and more. These alerts can be received in the form of an email, SMS, or phone call.
The value of a cloud-based monitoring solution is the assurance that the stored goods are being monitored 24/7, which far outweighs the initial cost of the data logger and the expense of the person to administer the data logger.
US Department of Health and Human Services, Centers for Disease Control and Prevention (2020). Vaccine Storage and Handling Toolkit. Retrieved from https://www.cdc.gov/vaccines/hcp/admin/storage/toolkit/storage-handling-toolkit-2020.pdf